Brain Imaging Study of the Impact of Sensory Enrichment in Autism
Researchers at the University of Wisconsin–Madison will measure changes in brain structure and in everyday skills and independence in autistic children and teens after 6 months of Sensory Enrichment Therapy, compared to standard of care, including ABA.
About the Study
This new research project explores how daily sensory and motor experiences may shape brain development in autism.
Using advanced MRI brain imaging, researchers aim to understand whether sensory enrichment—the same approach that improved outcomes in previous clinical trials—also produces measurable changes in brain structure.
The study builds on more than a decade of work showing that environmental enrichment can help the brain form new connections and support cognitive and emotional growth.
Why it matters
This is the first randomized controlled study designed to test structural brain change after a caregiver-implemented enrichment program in autistic youth.
Parenting a child with autism comes with many unknowns.
This study gives families the chance to contribute to research that could improve understanding of how the autistic brain can change.
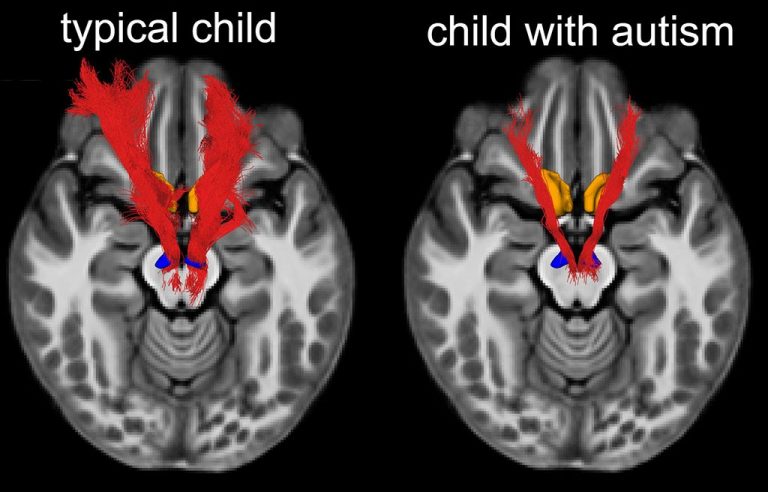
While some autism therapies have shown changes in brain activity using EEG or fMRI scans, there is still very little direct evidence that these approaches can change the structure of the brain itself (Calderoni et al., 2016; Dawson et al., 2012).
Previous studies of Sensory Enrichment Therapy (SET) showed strong clinical improvements in children with autism, but no brain imaging was done to see how the brain might have changed. (Woo & Leon, 2013; Woo et al., 2015).
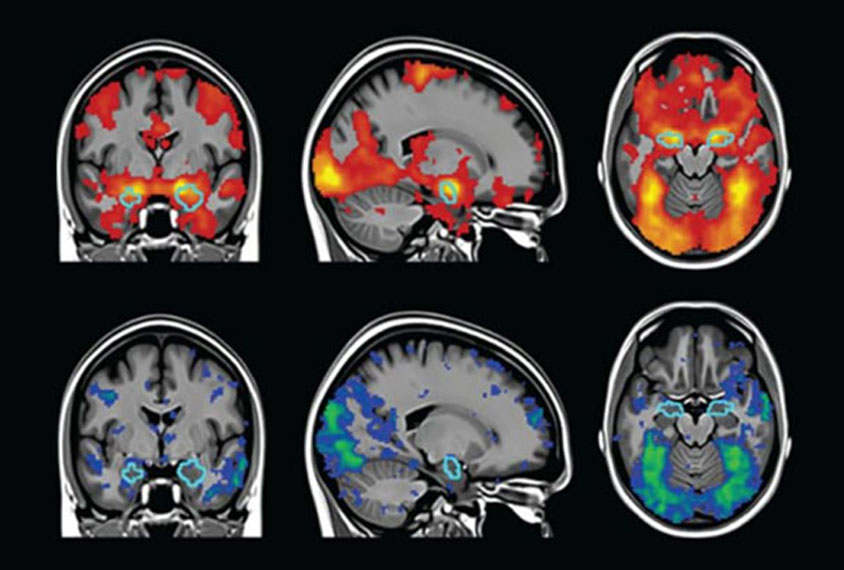
Researchers will use MRI to measure whether the thickness of certain brain regions—those involved in sensation, self-regulation, and planning—changes after 24 weeks of daily enrichment activities.
If the study finds that improvements in behavior are matched by measurable brain changes, it would show that SET directly influences how the brain develops and provide key data needed to design a larger, definitive trial.
About Sensory Enrichment Therapy
Research in neuroscience has shown that the brain grows and changes when it is exposed to rich, varied experiences (Nithianantharajah & Hannan, 2006; Rosenzweig & Bennett, 1996).
When animals live in environments with more sights, sounds, textures, movement, and opportunities to explore, their brains develop stronger connections between nerve cells.
These experiences help new synapses form, increase the number and size of brain branches, and even lead to a thicker outer layer of the brain—the cortex—where many higher-level functions happen.
In animal models of autism, this type of environmental enrichment has been shown to reduce autism-like behaviors and restore balance in brain circuits related to social interaction, emotion, and learning. This gives scientists confidence that similar approaches might benefit humans as well (Sale et al., 2014).
Sensory Enrichment Therapy (SET) takes these neuroscience principles and turns them into simple, structured sensory and motor activities that families can do at home.
Prior randomized studies of Sensory Enrichment Therapy reported improvements on standardized measures in autistic children.
This study is designed to evaluate brain MRI changes and link them to clinical measures. Individual results may vary; participation may not provide direct benefit.
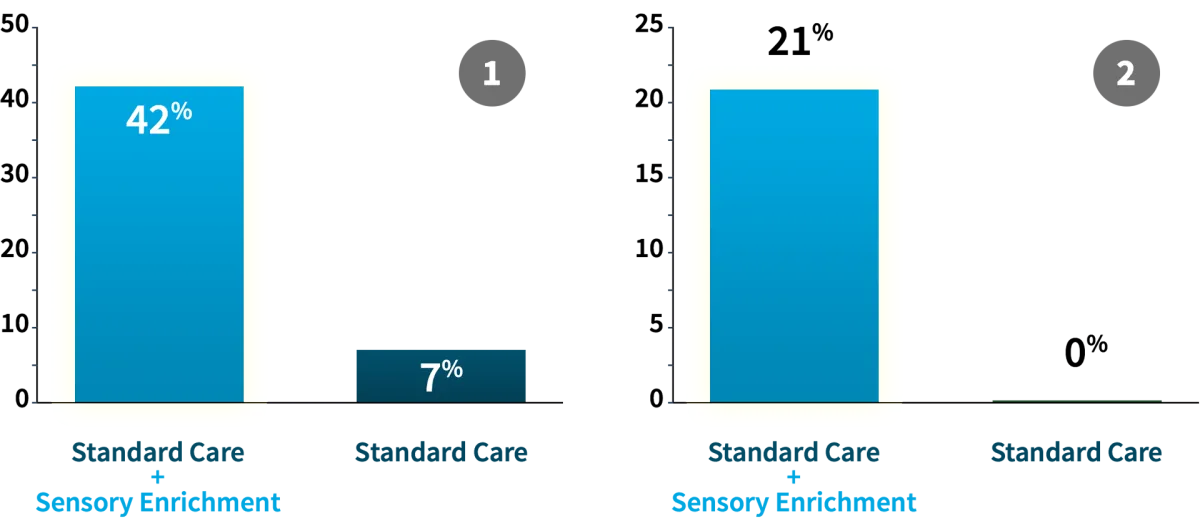
6 times more likely to make significant clinical progress compared to controls (Woo & Leon, Behavioral Neuroscience, 2013) (1)
An average gain of 11 IQ points in 6 months
21% of participants no longer qualified for initial diagnosis (ADOS) (Woo & Leon, Behavioral Neuroscience, 2015) (2)
Meaningful improvements in sensory processing, sleep, social engagement, speech, food diversification, mood, and behavior (Aronoff, Hillyer & Leon, Neural Plasticity, 2016)
Examples of Sensory Enrichment Games at Home

Eligibility
Inclusion criteria
You are eligible to participate if:
Diagnosis of Autism Spectrum Disorder (ASD)
Age between 8 and 18 years
Ability to undergo MRI scanning without sedation
MRI scan time is typically up to about 60 minutes (with breaks as needed). They are loud, but they are non-invasive and use no radiation. Children can wear ear protection, and parents are nearby during the scan.
English-speaking families or access to translation support
Study materials and assessments (e.g., CARS-2) are validated in English; comprehension ensures accuracy in data collection.
Agreement to attend all study visits
The study involves two in-person visits (at the beginning and end) at the University of Wisconsin–Madison MRI facility. Each visit lasts about 3 hours, to participate in an MRI session and CARS test with a psychometrician. Travel costs, parking, or time MAY be reimbursed (discuss during your interview).
Agreement to complete surveys during the study
We also require you to complete 5 surveys at the beginning, the middle and the end of the study. They will be provided during the intake visit. It should take you about 1 hour each time to complete the surveys.
Exclusion criteria
You may not participate if:
Genetic or syndromic forms of autism (e.g., Rett syndrome, Fragile X, Down syndrome)
These conditions can cause distinct brain and developmental profiles unrelated to autism.
Seizure disorder not well controlled by medication
To ensure participant safety during MRI sessions and prevent data artifacts caused by seizure activity.
Started new medication less than 8 weeks before enrollment
Medication or therapy changes can affect brain function and behavior, making it difficult to measure the specific effects of sensory enrichment.
Metal implants or devices incompatible with MRI
Devices, like braces, cochlear implants, surgical clips may cause injury to the participant during the MRI session or data distortion.
Severe claustrophobia or anxiety preventing MRI
MRI requires participants to stay in an enclosed scanner for up to 10 minutes at a time; severe anxiety could interfere with completion of imaging.
Current participation in another clinical trial
Prevents interference or confounding from other interventions.
Major medical or psychiatric conditions affecting brain function
Conditions like traumatic brain injury, schizophrenia, major depression could independently alter neural patterns and confound imaging data.Unwillingness or inability of the family to commit to daily sensory enrichment activities for six months
You will be testing Sensory Enrichment Therapy at home. The program consists in brain stimulation games that you play as a family, using typical household items. Your total time commitment is for 15 minutes per session, for a total of 2 sessions per day. You will also be meeting with a coach online for 30 minutes every week.
How to Join
1. Complete the short intake form to determine eligibility
2. If eligible, a research coordinator will contact you to schedule your MRI visit and walk you through the program
3. If eligible, you will receive the Sensory Enrichment Therapy program at no cost for research purposes and follow it from home for 6 months
Timeline
Enrollment open between January 2026 and January 2027.
Participation length: About 6 months from your first visit.
Visits:
Visit 1 (Month 0): Baseline MRI and behavioral assessments (about 3 hours) at the University of Wisconsin and the Waisman Center in Madison.
Home activities: Simple games you play as a family for a total of approximately 30 minutes per day, for 6 months, using typical household items.
Weekly remote coaching sessions: 30 minutes per week, using video conferencing.
Surveys at home: Completed online at start and end (6 months). 1 hour to complete surveys.
Visit 2 (Month 6): Follow-up MRI and final assessments (about 3 hours).
Results and updates: Participants will receive study updates by email once data collection is complete.
Travel costs, parking, or time MAY be reimbursed. Please discuss with the study coordinator during your interview.
Risks
This is a research study, not medical treatment
Risks include:
MRI discomfort (noise, confined space) and survey fatigue; we provide mock-MRI practice and child-friendly supports.
Sensory Enrichment Therapy may lead on a short and temporary basis to mild mood fluctuations, sleep disruptions and increased urination.
Participation is voluntary and you may stop at any time.
You may not receive direct benefit.
We protect your privacy and use your contact information only to respond about this study.
Ethics and study partners
IRB # [TBD]
This research is conducted by the University of Wisconsin–Madison Department of Radiology, under the direction of Dr. Vivek Prabhakaran, in collaboration with the Waisman Center.
IRB approval pending
Mendability supports the study by providing access to the sensory enrichment intervention at no cost to participants.
Contact
Principal investigator: Vivek Prabhakaran, MD, PhD.
Direct inquiries to: Anusha Adluru, [email protected], 608 512 3275.